PAIN – from phenotypes to mechanisms
Project leader: Prof. Dr. Michèle Hubli
Interpatient variability in the outcomes of pain treatment is impressively large and has led to calls for “precision medicine” employing pain phenotyping. Beyond the clinical phenotyping, a multi-modal neurophysiological approach serves as an objective measure of afferent integrity yielding insights into nociceptive processing and signal integration at several neural levels.
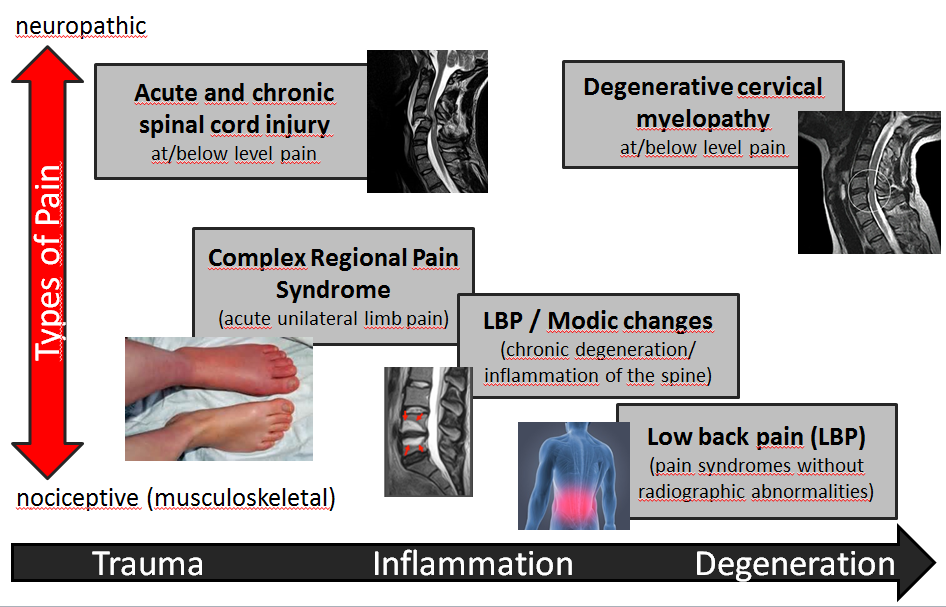
The main research question is in how far we can stratify patients across different chronic pain entities (see Figure 1) according to their distinct pain phenotype. This is achieved by deconstructing the clinical phenotype and attribute clinical signs and symptoms of the chronic pain condition to either common or disease-specific pathophysiological processes. The clinical presentation of chronic pain conditions will likely show distinct but also common pattern of sensory abnormalities, integrity of residual afferent tracts, nociceptive processing, as well as sensory-autonomic interaction.
Figure 1: Spectrum of pain disorders. Patients across different chronic pain entities (from neuropathic to nociceptive pain) can be stratified according to their distinct pain phenotype. This will be assessed using a common test battery including clinical phenotyping and multimodal neurophysiology.
Project partners:
PD Dr. Stefan Dudli, Center of Experimental Rheumatology, Balgrist University Hospital and University Hospital Zurich
Prof. Dr. Catherine Jutzeler, Biomedical Data Science Lab, D-HEST, ETH Zurich
Prof. Dr. Oliver Distler, Department of Rheumatology, University Hospital Zurich
Prof. Dr. Mazda Farshad, Department of Orthopedics, Balgrist University Hospital
PD Dr. Florian Brunner, Department of Physical Medicine and Rheumatology, Balgrist University Hospital
Dr. Petra Schweinhardt, Department of Chiropractic, Balgrist University Hospital
Funding:
Clinical Research Priority Program PAIN 2019-2021, 2022-2024, University of Zurich
Publications:
- De Schoenmacker I, Costa Marques D, Scheuren PS, Lütolf R, Gorrell LM, Mehli SC, Curt A, Rosner R, Hubli M. Novel neurophysiological evidence for preserved pain habituation across chronic pain conditions. Clin Neurophysiol. 2024 Oct: 166:31-42.
- Gozzi N, Preatoni G, Ciotti F, Hubli M, Schweinhardt P, Curt A, Raspopovic S. Unravelling the physiological and psychosocial signatures of pain by machine learning. Med. 2024 Aug 1: S2666-6340(24)00298-8.
- Sirucek L, De Schoenmacker I, Scheuren PS, Lütolf R, Gorell LM, Langenfeld A, Baechler M, Rosner J, Wirth B, Hubli M, Schweinhardt P. Indication for spinal sensitization in chronic low back pain: mechanical hyperalgesia adjacent to but not within the most painful body area. Pain Rep. 2024 Jun 17;9(4):e1166.
- Allmendinger F, Scheuren PS, De Schoenmacker I, Brunner F, Rosner J, Curt A, Hubli M. Contact-Heat Evoked Potentials: Insights into Pain Processing in CRPS Type I. J Pain Res. 2024 Mar 13;17:989-1003.
- De Schoenmacker I, Sirucek L, Scheuren PS, Lütolf R, Gorell LM, Brunner F, Curt A, Rosner J, Schweinhardt P, Hubli M. Sensory phenotypes in complex regional pain syndrome and chronic low back pain - indication of common underlying pathomechanisms. Pain Rep. 2023 Nov;15;8(6):e1110.
- De Schoenmacker I, Mollo A, Scheuren PS, Sirucek L, Brunner F, Schweinhardt P, Curt A, Rosner J, Hubli M. Central sensitization in CRPS patients with a widespread pain: a cross-sectional study. Pain Med. 2023 Aug 1;24(8):974-984.
- Scheuren PS, De Schoenmacker I, Rosner J, Brunner F, Curt A, Hubli M. Pain-autonomic measures reveal nociceptive sensitization in complex regional pain syndrome. Eur J Pain. 2023 Jan;27(1):72-85.
For further information please contact: Prof. Dr. Michèle Hubli