Impact of deafferentation on descending pain control systems
Project leader: Prof. Dr. Michèle Hubli
Three projects focus on alterations of the sensori-sensory interactions and the descending pain control systems following deafferentation in individuals with spinal cord injury (SCI) using clinical, neurophysiological, and neuro-imaging assessments. The overall aim is to improve our understanding of how the descending control of afferent stimuli is altered in response to deafferenation (i.e., changes in the modulation of innocuous and nociceptive thermal inputs) and the evolution of changes throughout the course of disease.
The overall objective is tackled with three projects that incorporate neurophysiological and neuroimaging techniques applied in healthy controls and individuals with SCI:
In project 1, the specificity and sensitivity of cold-evoked potentials will be assessed in healthy controls and individuals with chronic SCI. Subsequently, in the framework of a longitudinal study in individuals with acute SCI we will investigate the responsiveness of the cold-evoked potentials (CEPs ) and contact-heat evoked potentials (CHEPs). Furthermore, the modulation of CEPs by means of thermal conditioning (i.e., cold, warm, and interlaced cold/warm) is investigated in healthy controls to reveal the impact of concomitant afferent inputs on the sensory processing.
Project 2 focuses on the impact of deafferentation on the supra-spinal and spinal endogenous capacity to modulate afferent inputs will be examined in individuals with SCI (i.e., above the level of injury) compared to controls. Employing conditioning pain modulation (CPM, see Figure 1) paradigm and the application of large fiber mediated stimulation (i.e., with TENS) will assess the interaction between CPM and non-painful afferent inputs on descending pain control. Importantly, the capacity to modulate afferent inputs following SCI will be a dynamic process that evolves from acute to chronic SCI and is likely influenced (or underlying) by the development of neuropathic pain.
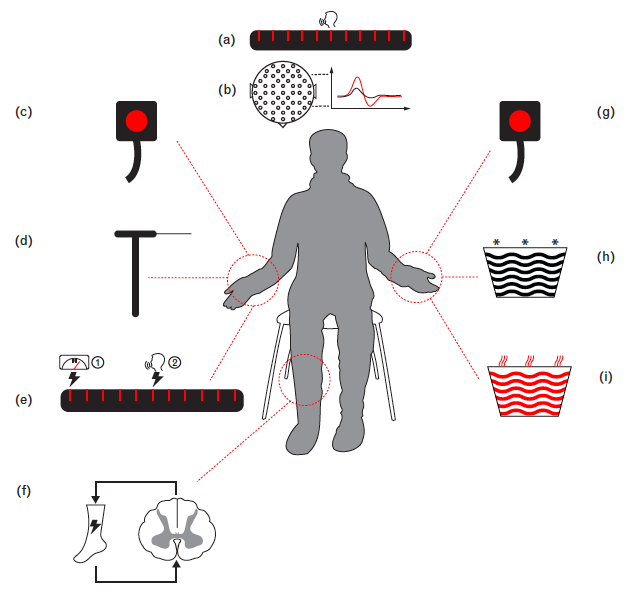
Figure 1: Schematic illustration of CPM paradigm. CPM effect is expressed by the reduced painfulness of the test stimulus induced by the application of a heterotopic conditioning stimulus. Representative test stimuli include thermal, mechanical, electrical modalities, while typical conditioning stimuli primarily consist of thermal stimuli, e.g. heat or cold. The CPM effect can be measured by either subjective numerical pain ratings, or objective features of pain-evoked potentials (from Nir and Yarnitsky, 2015).
Lastly, project 3 intents to identify cerebral areas mediating descending inhibitory action as well as to reveal potential changes in these areas which might be responsible for the altered capacity to modulate afferent input. CPM and large A-beta fiber-mediated pain modulation will be used to engage pain control networks. The combination of structural (gray and white matter properties) with evoked brain responses, resting-state activity, and connectivity (Figure 3) multimodal neuroimaging will provide complimentary information for elucidating mechanisms of altered sensory perceptions, which could provide future directions for treatment.
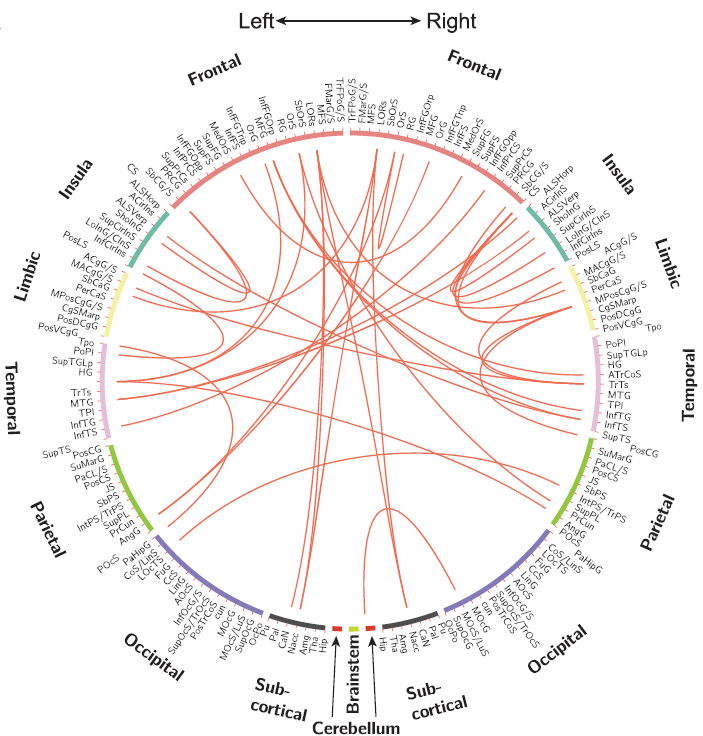
Figure 2: Example of functional brain connectivity. Red lines indicate pairs or regions where functional connectivity increased in patients with more widespread pain compared to localized pain (from Kutch et al., 2017).
Publications:
- Huynh V, Luetolf R, Rosner J, Luechinger R, Kollias S, Curt A, Michels L, Hubli M*. Intrinsic brain connectivity differentiates intact endogenous pain modulation in subjects with neuropathic pain after spinal cord injury. Sci Rep. 2023 Jul 24;13(1):11943. (*shared last authorship).
- Lütolf R., De Schoenmacker I, Rosner J, Sirucek L, Schweinhardt P, Curt A, Hubli M. Anti- and Pro-Nociceptive mechanisms in neuropathic pain after human spinal cord injury. Eur J Pain. 2022 Nov;26(10):2176-2187.
- Huynh V, Luetolf R, Rosner J, Luechinger R, Curt A, Kollias S, Michels L, Hubli M*. Descending pain modulatory efficiency in healthy subjects is related to structure and resting connectivity of brain regions. Neuroimage. 2022 Feb 15; 247:118742. (*shared last authorship).
- Scheuren P, Nauer N, Rosner R, Curt A, Hubli M. Cold evoked potentials elicited by rapid cooling of the skin across different age groups and stimulation sites. Sci Rep. 2022 Mar 9;12(1):4137.
- Huynh V, Luetolf R, Rosner J, Luechinger R, Curt A, Kollias S, Hubli M*, Michels L. Supraspinal Nociceptive Networks in Neuropathic Pain after Spinal Cord Injury. Hum Brain Mapp. 2021 Aug 15;42(12):3733-3749. (*shared last authorship).
- Huynh V, Staempfli P, Luetolf R, Luechinger R, Curt A, Kollias S, Hubli M*, Michels L. Investigation of Cerebral White Matter Changes After Spinal Cord Injury With a Measure of Fiber Density. Front Neurol. 2021 Feb 22;12:598336. (*shared last authorship).
- Rosner J, Rinert J, Ernst M, Curt A, Hubli M. Cold evoked potentials: Acquisition from cervical dermatomes. Neurophys Clin. 2019 Feb;49(1):49-57.
External project partners:
Prof. Dr. Spyros Kollias, Institute of Neuroradiology, University Hospital Zurich, Switzerland
PD Dr. Lars Michels, Institute of Neuroradiology, University Hospital Zurich, Switzerland
Dr. Kip Kramer, ICORD, University of British Columbia, Canada
Dr. Catherine Jutzeler, ICORD, University of British Columbia, Canada
Funding:
Swiss National Science Foundation
Swiss Spinal Cord Injury Cohort Study Nested Project Grant
Hartmann Müller-Stiftung für Medizinische Forschung
For further information please contact: Prof. Dr. Michèle Hubli