Neural Stem Cell Trial (study terminated - no more participants)
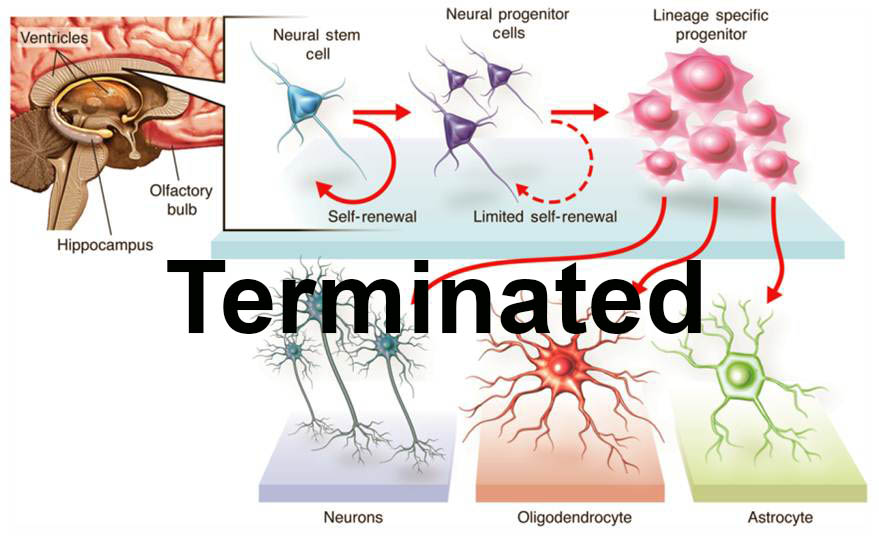
The trial did enroll 12 patients with thoracic (chest-level) spinal cord injury who have a neurological injury level of T2-T11, and included both complete and incomplete injuries as classified by the American Spinal Injury Association (ASIA) Impairment Scale. The first cohort were patients classified as ASIA A, or patients who have what is considered to be a "complete" injury, or no movement or feeling below the level of the injury. The second cohort did progress to patients classified as ASIA B, or patients with some degree of feeling below the injury. The third cohort consisted of patients classified as ASIA C, or patients with some degree of movement below the injury. In addition to assessing safety, the trial did measure defined clinical endpoints, such as changes in sensation, motor, and bowel/bladder function. All patients received HuCNS-SC cells through direct transplantation into the spinal cord, and were temporarily immunosuppressed. Following transplantation, the patients were evaluated regularly over a 12-month period in order to monitor and evaluate the safety and tolerability of the HuCNS-SC cells, the surgery and the immunosuppression, and to measure any recovery of neurological function below the injury site. As the Company intends to follow the effects of this therapy long-term, a separate four-year observational study was initiated at the conclusion of this trial.
The trial is initiated by StemCells, Inc. Palo Alto, California, and is being conducted at the Balgrist University Hospital, University of Zurich, Switzerland.
Publication
- Curt A, The Damaged Spinal Cord Is a Suitable Target for Stem Cell Transplantation. DOI: 10.1177/1545968320935815