Navigation auf uzh.ch
Navigation auf uzh.ch
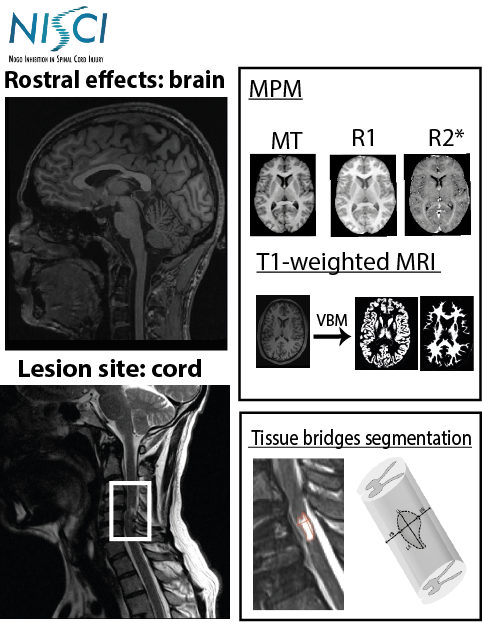
This project aims to apply a novel qMRI protocol covering brain and cervical cord down to C4 level as a sub-study within the clinical trial NISCI (www.nisci-2020.eu) to identify iron and myelin-changes in the spinal cord and brain and correlate them to the level of deficit and functional improvement of the patients in the two treatment groups (ATI355 vs. placebo).
Biomarkers for the NISCI trial
The NISCI clinical trial is a state-of-the-art placebo-controlled multicentric phase II clinical trial in a consortium of seven leading European SCI centers to assess the efficacy of anti-Nogo-A antibody therapy to significantly improve the neurological recovery and functional outcome of spinal cord injured patients.
The multi-parameter mapping has been optimized for the application on clinical 3T scanners based on product sequences (1 mm isotropic resolution, scanning time <25 min) (Leutritz et al., 2020, HBM). To evaluate the protocol setup for consistency between and within sites (test-retest) we performed a traveling heads study with five healthy subjects across six sites, involving different scanner hard- and software with five healthy subjects across six sites, involving different scanner hard- and software (Leutritz et al., 2020, HBM, 41:42 32– 4247).
For processing the data we used the hMRI-toolbox (www.hmri.info) for quantitative MRI data, which is developed by the MPI-CBS and an international consortium (Tabelow et al., 2019, NeuroImage, 194, 191-210). This study is supported by Horizon 2020 Framework Programme, 681094.